Presenter Name: Hayden Johns
Description
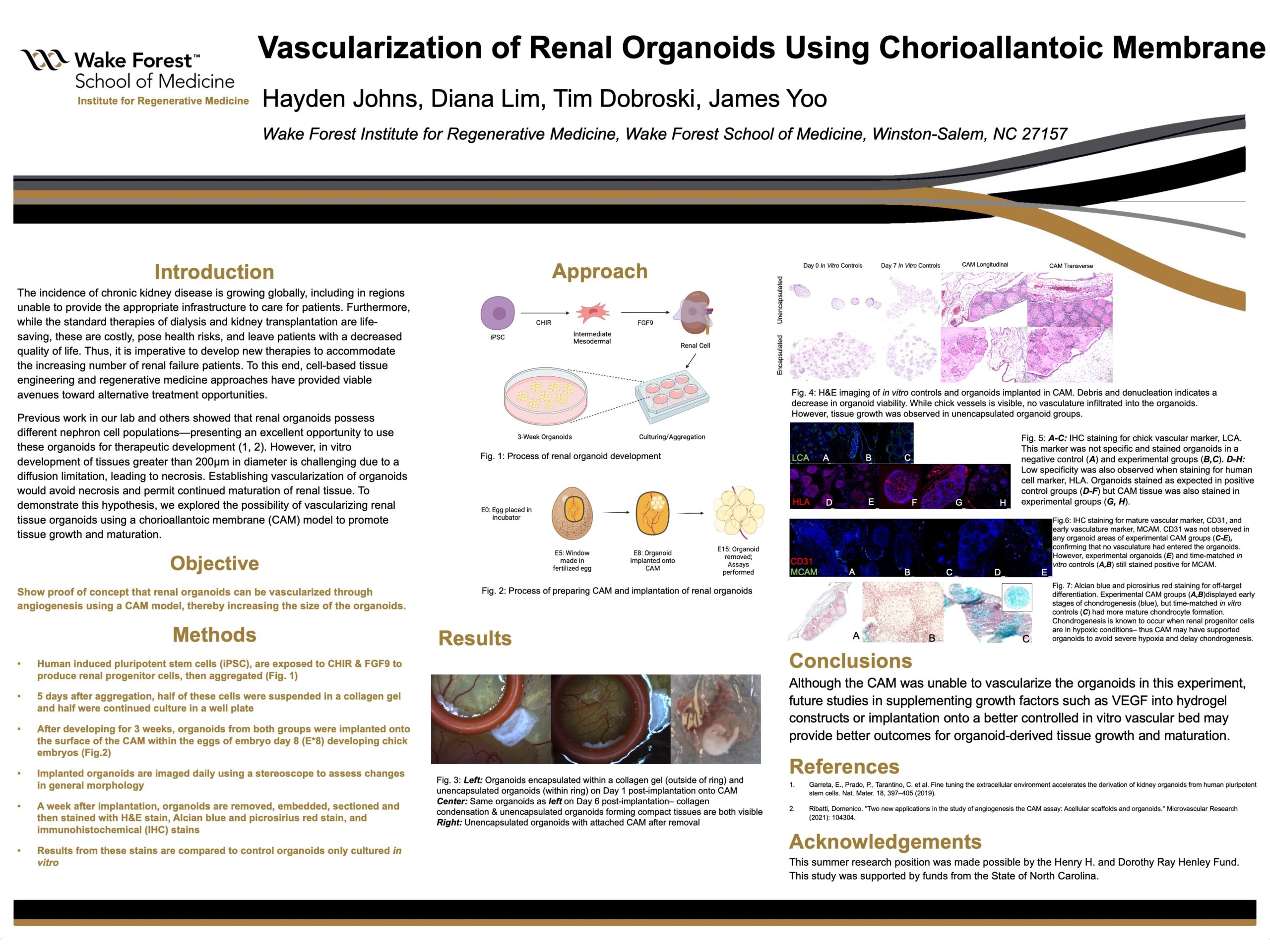
The incidence of chronic kidney disease is growing globally, including in regions unable to provide the appropriate infrastructure to care for patients. Furthermore, while the standard therapies of dialysis and kidney transplantation are life-saving, these are costly, pose health risks, and leave patients with a decreased quality of life. Thus, it is imperative to develop new therapies to accommodate the increasing number of renal failure patients. To this end, cell-based tissue engineering and regenerative medicine approaches have provided viable avenues toward alternative treatment opportunities.
Previous work in our lab and others showed that renal organoids possess different nephron cell populations, including proximal tubule, loop of Henle, distal tubule, and glomeruli-presenting an excellent opportunity to use these organoids for therapeutic development. Although utilizing the renal organoids to generate kidney tissues is a potential solution, in vitro development of large tissues greater than 200µm in diameter is challenging due to a diffusion limitation, leading to necrosis. Establishing vascularization of organoids would avoid necrosis and permit continued maturation of renal tissue. To demonstrate this hypothesis, we explored the possibility of vascularizing renal tissue organoids using a chorioallantoic membrane (CAM) model to promote tissue growth and maturation.
Renal organoids were derived from induced pluripotent stem cells using differentiation factors (CHIR and FGF9). After differentiation, cells were aggregated and matured into organoids for five days in vitro. A subset of these organoids was encapsulated in a collagen hydrogel and incubated for culture, while the remainder were cultured as free organoids for three weeks. The organoids were implanted onto the CAM of 5-day chick eggs, and images were taken daily with a stereoscope to evaluate morphological changes. After seven days in ovo, the organoid samples were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E) stain to assess tissue morphology. Alcian blue and picrosirius red stains were performed to detect off-target differentiation, such as chondrogenesis. Immunohistochemistry (IHC) was performed to identify the early and mature vascular markers, MCAM and CD31, respectively.
After analysis we found that endothelial cells were not present within the organoids-suggesting no vascularization had occurred-but unencapsulated organoids implanted onto the CAM did grow into larger tissues. However, all groups of organoids showed evidence of off-target chondrogenesis, likely due to the hypoxic conditions generated by this lack of vascularization and integration. This finding demonstrates that the CAM may have supported the organoids enough to delay severe hypoxia. Although the CAM was unable to vascularize the organoids in this experiment, future studies in supplementing growth factors such as VEGF into hydrogel constructs or implantation onto a better controlled in vitro vascular bed may provide better outcomes for organoid-derived tissue growth and maturation.
Previous work in our lab and others showed that renal organoids possess different nephron cell populations, including proximal tubule, loop of Henle, distal tubule, and glomeruli-presenting an excellent opportunity to use these organoids for therapeutic development. Although utilizing the renal organoids to generate kidney tissues is a potential solution, in vitro development of large tissues greater than 200µm in diameter is challenging due to a diffusion limitation, leading to necrosis. Establishing vascularization of organoids would avoid necrosis and permit continued maturation of renal tissue. To demonstrate this hypothesis, we explored the possibility of vascularizing renal tissue organoids using a chorioallantoic membrane (CAM) model to promote tissue growth and maturation.
Renal organoids were derived from induced pluripotent stem cells using differentiation factors (CHIR and FGF9). After differentiation, cells were aggregated and matured into organoids for five days in vitro. A subset of these organoids was encapsulated in a collagen hydrogel and incubated for culture, while the remainder were cultured as free organoids for three weeks. The organoids were implanted onto the CAM of 5-day chick eggs, and images were taken daily with a stereoscope to evaluate morphological changes. After seven days in ovo, the organoid samples were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E) stain to assess tissue morphology. Alcian blue and picrosirius red stains were performed to detect off-target differentiation, such as chondrogenesis. Immunohistochemistry (IHC) was performed to identify the early and mature vascular markers, MCAM and CD31, respectively.
After analysis we found that endothelial cells were not present within the organoids-suggesting no vascularization had occurred-but unencapsulated organoids implanted onto the CAM did grow into larger tissues. However, all groups of organoids showed evidence of off-target chondrogenesis, likely due to the hypoxic conditions generated by this lack of vascularization and integration. This finding demonstrates that the CAM may have supported the organoids enough to delay severe hypoxia. Although the CAM was unable to vascularize the organoids in this experiment, future studies in supplementing growth factors such as VEGF into hydrogel constructs or implantation onto a better controlled in vitro vascular bed may provide better outcomes for organoid-derived tissue growth and maturation.
University / Institution: Utah State University
Type: Poster
Format: In Person
Presentation #D4
SESSION D (3:30-5:00PM)
Area of Research: Health & Medicine
Email: hayden.johns@usu.edu
Faculty Mentor: Justin Jones